Thanks to an experimental new therapy, a seven-year-old girl in the US with
an aggressive form of childhood leukemia has been cured with her own
re-engineered immune cells. After the treatment, her doctors could find no
evidence of cancer.
Pediatric oncologist Stephan A Grupp, of The Children's Hospital of Philadelphia (CHOP), and his team, were able to help Emily Whitehead by recruiting her as the first child patient on a clinical trial he is leading called CTL019, which is testing T cell therapy to treat acute lymphoblastic leukemia (ALL) and other B cell cancers.
On the trial, Emily (also known as Emma), who had relapsed twice following standard chemotherapy for ALL, received a custom-designed version of her own T cells which rapidly multiplied and destroyed the leukemia cells.
Grupp is the director of Translational Research for the Center for Childhood Cancer Research at CHOP, and professor of Pediatrics at the Perelman School of Medicine at the University of Pennsylvania.
He and his team from CHOP and the University of Pennsylvania presented the updated trial results at the 2012 annual meeting of the American Society of Hematology (ASH), which took place in Atlanta from 8 to 11 December.
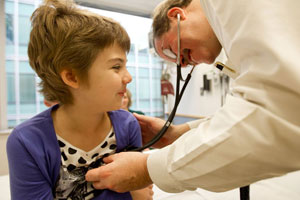
Dr. Stephan Grupp, M.D., Ph.D. of The Children's Hospital of Philadelphia examines first pediatric patient Emma six months after T Cell therapy. Emma had relapsed B cell leukemia.
In the trial,
researchers took immune system T cells from each patient's own blood. These
cells are often called the "workhorses" of the immune system, they do the "heavy
work" of recognizing and attacking invading disease cells.
The team then genetically modified the extracted T cells to express a protein that binds to a target called CD19, a protein found on the surface of B cells, a type of white blood cell that becomes cancerous in certain leukemias and lymphomas, such as ALL, NHL and CLL.
When they become cancerous, B cells are capable of "flying under the radar" of the T cells and evade them. The genetic modification makes T cells capable of detecting and then destroying the cancerous B cells. The genetically reengineered T cells are referred to as "chimeric antigen receptor T cells".
After reengineering, the modified T cells are then put back into the patient where they disperse and go in search of the cancerous B cells. They also multiply in the body at a rate that enables them to kill the rapidly dividing cancerous B cells. And they also stay in the body and continue to fight any new cancerous B cells.
For instance, the modified T cells can survive for many months in the patient's body, and have the ability to grow into large quantities. And they have killed large quantities of ALL or CLL cells in 9 of the 12 patients.
The team hopes CTL019 will prove an effective therapy for patients with B cell cancers. But because only a few patients are on this phase of the trial, there now needs to be a big push to enrol many more adult and pediatric patients, to see if the same results are seen in a larger group.
At first Emily's doctors thought she would be among the 85% with the type of ALL that would respond to chemo, but a relapse proved she had the more resistant variety.
After a second relapse following a second course of chemo, Emily was enrolled on the CTL019 clinical trial.
Unfortunately, Emily got very ill during the trial. While flu-like symptoms were expected, Emily developed cytokine release syndrome: the effect of T cells growing in Emily's body caused high levels of a certain protein that is involved in rheumatoid arthritis.
After administering a drug to counteract the protein, Emily's condition improved dramatically: almost overnight her breathing improved, her fever dropped, and her blood pressure went back to normal.
In the weeks that followed, Emilly recovered completely from the cytokine release syndrome, but her doctors weren't sure if the T cell therapy was working. So they did a bone marrow test to check for results.
Grupp takes up the story:
"Three weeks after receiving the treatment, she was in remission. Emily completely responded to her T cell therapy."
"We checked her bone marrow for the possibility of disease again at three months and six months out from her treatment, and she still has no disease whatsoever. The cancer-fighting T cells are still there in her body," he adds.
It is for cancers that have not responded to standard chemotherapy: it is not for people with newly diagnosed disease.
The researchers warn that these results are still preliminary, and that not all children who qualify for the trial will have the same result.
In the long term, Grupp sees cell therapies like CTL019 as potential replacements for bone marrow transplants.
"I've been meeting with families to discuss bone marrow transplant for 20 years," he says.
"In almost every meeting, I say that bone marrow transplant is very hard and that if we had an alternative for children at that point in treatment, I would be delighted to put myself out of business. And for the first time, we're seeing how that might actually happen."
Pediatric oncologist Stephan A Grupp, of The Children's Hospital of Philadelphia (CHOP), and his team, were able to help Emily Whitehead by recruiting her as the first child patient on a clinical trial he is leading called CTL019, which is testing T cell therapy to treat acute lymphoblastic leukemia (ALL) and other B cell cancers.
On the trial, Emily (also known as Emma), who had relapsed twice following standard chemotherapy for ALL, received a custom-designed version of her own T cells which rapidly multiplied and destroyed the leukemia cells.
Grupp is the director of Translational Research for the Center for Childhood Cancer Research at CHOP, and professor of Pediatrics at the Perelman School of Medicine at the University of Pennsylvania.
He and his team from CHOP and the University of Pennsylvania presented the updated trial results at the 2012 annual meeting of the American Society of Hematology (ASH), which took place in Atlanta from 8 to 11 December.
How T Cell Therapy Works on the Clinical Trial CTL019
The T Cell Therapy Clinical Trial CTL019 (formerly CART19) is testing the effect of T cell therapy for patients with B cell cancers such as acute lymphoblastic leukemia (ALL), B cell non-Hodgkin lymphoma (NHL), and the adult disease chronic lymphocytic leukemia (CLL).
Dr. Stephan Grupp, M.D., Ph.D. of The Children's Hospital of Philadelphia examines first pediatric patient Emma six months after T Cell therapy. Emma had relapsed B cell leukemia.
The team then genetically modified the extracted T cells to express a protein that binds to a target called CD19, a protein found on the surface of B cells, a type of white blood cell that becomes cancerous in certain leukemias and lymphomas, such as ALL, NHL and CLL.
When they become cancerous, B cells are capable of "flying under the radar" of the T cells and evade them. The genetic modification makes T cells capable of detecting and then destroying the cancerous B cells. The genetically reengineered T cells are referred to as "chimeric antigen receptor T cells".
After reengineering, the modified T cells are then put back into the patient where they disperse and go in search of the cancerous B cells. They also multiply in the body at a rate that enables them to kill the rapidly dividing cancerous B cells. And they also stay in the body and continue to fight any new cancerous B cells.
Initial Results Show Promise
Only 12 patients have been treated so far: 10 adults with chronic lymphocytic leukemia (CLL), and 2 children with acute lymphoblastic leukemia (Emily being one of them). But researchers say the early results show great promise.For instance, the modified T cells can survive for many months in the patient's body, and have the ability to grow into large quantities. And they have killed large quantities of ALL or CLL cells in 9 of the 12 patients.
The team hopes CTL019 will prove an effective therapy for patients with B cell cancers. But because only a few patients are on this phase of the trial, there now needs to be a big push to enrol many more adult and pediatric patients, to see if the same results are seen in a larger group.
Emily's Story
85% of children with ALL, the most common childhood cancer, are cured after two years of treatment with standard chemotherapy. But there is a 15% minority that has a type of ALL that resists even the most intense chemo regimens.At first Emily's doctors thought she would be among the 85% with the type of ALL that would respond to chemo, but a relapse proved she had the more resistant variety.
After a second relapse following a second course of chemo, Emily was enrolled on the CTL019 clinical trial.
Unfortunately, Emily got very ill during the trial. While flu-like symptoms were expected, Emily developed cytokine release syndrome: the effect of T cells growing in Emily's body caused high levels of a certain protein that is involved in rheumatoid arthritis.
After administering a drug to counteract the protein, Emily's condition improved dramatically: almost overnight her breathing improved, her fever dropped, and her blood pressure went back to normal.
In the weeks that followed, Emilly recovered completely from the cytokine release syndrome, but her doctors weren't sure if the T cell therapy was working. So they did a bone marrow test to check for results.
Grupp takes up the story:
"Three weeks after receiving the treatment, she was in remission. Emily completely responded to her T cell therapy."
"We checked her bone marrow for the possibility of disease again at three months and six months out from her treatment, and she still has no disease whatsoever. The cancer-fighting T cells are still there in her body," he adds.
Who Else Might Benefit from the Treatment Emily Received?
The team points out that CTL019 is designed for children and teenagers with fairly advanced B cell acute lymphoblastic leukemia (ALL) and B cell lymphomas only.It is for cancers that have not responded to standard chemotherapy: it is not for people with newly diagnosed disease.
The researchers warn that these results are still preliminary, and that not all children who qualify for the trial will have the same result.
In the long term, Grupp sees cell therapies like CTL019 as potential replacements for bone marrow transplants.
"I've been meeting with families to discuss bone marrow transplant for 20 years," he says.
"In almost every meeting, I say that bone marrow transplant is very hard and that if we had an alternative for children at that point in treatment, I would be delighted to put myself out of business. And for the first time, we're seeing how that might actually happen."
No comments:
Post a Comment